
It is now quite clear that DNA copies of viral RNA are made in infected cells. A DNA copy of lymphocytic choriomeningitis virus (LCMV) RNA has been detected in mouse cells, and this DNA can be integrated into cellular DNA. LCMV DNA was only detected in cells that produce a retrovirus, implicating reverse transcriptase in this process. DNAs of various RNA viruses, including bornaviruses and filoviruses, have been found integrated into the genome of many animals. An explanation for the genesis of these DNA copies is provided by studies of cells infected with vesicular stomatitis virus.
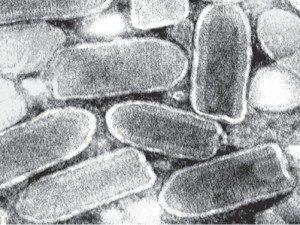
Vesicular stomatitis virus (pictured) is an enveloped virus with a genome of (-) strand RNA. In infected cells the (-) strand RNA is copied by the viral RNA polymerase to form 5 mRNAs which encode the viral proteins. Infection of various human cell lines resulted in the production of DNA complementary to VSV RNA. The DNAs are single stranded and appear to be produced from the viral mRNAs, not the viral genome.
How might VSV DNA be produced in virus infected cells? About 20% of the human genome consists of a mobile genetic element called LINE-1 (Long Interspersed Nuclear Element). These elements, also called retrotransposons, encode a reverse transcriptase. This enzyme converts LINE-1 mRNA into DNA, which can then integrate elsewhere in the cell genome – hence the name mobile genetic element. LINE-1 encoded reverse transcriptase can also produce DNA copies of cellular mRNAs and other mobile elements that do not encode their own reverse transcriptase. Thanks to LINEs, our genomes are littered with mobile genetic elements.
To determine if LINE-1 could make VSV in infected cells, the authors took advantage of their observation that not all human cells produce VSV DNA after infection. Introduction of LINE-1 DNA into one of these cell lines enabled it to produce DNA copies of VSV mRNAs. This result shows that LINE-1 can make VSV DNA in infected cells. Whether LINE-1 actually accomplishes this process in unmodified, VSV infected cells remains to be proven.
The authors also show that viral DNA is produced in cells infected with two other RNA-containing viruses, echovirus and respiratory syncytial virus. The latter of course is the virus used to make the claim nearly 40 years ago that viral DNA is present in infected cells. Whether that viral DNA is infectious, however, is not known. No complete copies of the VSV RNA genome have been observed in infected cells, only copies of viral mRNAs.
It seems likely that in cells infected with RNA viruses, reverse transcriptase encoded by LINE-1 could produce DNA copies of viral RNAs. After entering the nucleus these viral DNAs could become integrated into cellular DNA. If a germline cell were infected, the integrated viral DNA would be passed on to subsequent generations, explaining the presence of DNA copies of various RNA viruses in animal genomes.
A key question is whether the production of DNA copies of viral RNAs is an accident, or benefits the virus or host. There is yet no answer to this question. The authors suggest that DNA copies of viral RNA might contribute to innate immune sensing of viral infection. VSV replication is not altered by the presence or absence of viral DNA, but there could be a role for viral DNA during infection of a host animal.

Hi Dr. Racaniello,
Long time reader/listener. I’m a couple weeks away from defending my Ph.D dissertation and will be at ASV in Fort Collins (I’m a grad student at CSU) coming up in a few weeks. Looking forward to your podcast there.
In any case, my research involves arboviruses and mosquito vectors, so I thought I’d share a paper that relates to this post. Specifically, my Ph.D work is over the antiviral RNAi response in mosquitoes to arbovirus infection.
RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila.
http://www.ncbi.nlm.nih.gov/pubmed/23435119
In this paper, the authors found the presence of fragments of the Flock house virus genome integrated into Drosophila cells. Long story short, they found that the reverse transcriptase activity of LTR-retrotransposons was capable of reverse transcribing short fragments of the virus genome, and that the resulting viral DNA was either integrated into the cell genome or possibly exists as circularized extrachromosomal DNA. They also found evidence that these viral DNAs were transcriptionally active and could produce dsRNA that fed back into the siRNA pathway. They propose a model whereby this feedback loop results in an equilibrium that allows for a persistent infection to develop. Cool stuff.
Abhishek Prasad
Pingback: Unexpected viral DNA in RNA virus-infected cell...
Interesting stuff! If my iPad in rural Ireland will let me, I’ll take the liberty of commenting that there are a number of reasonably neglected reports on the presence of cDNA copies of all or part of viral genomes in host cell genomes, including for virus of honeybees, vertebrates and plants. It seems to be a reasonably common occurrence, and MUST be linked to some kind of survival advantage for the host. siRNA mediated resistance, possibly??