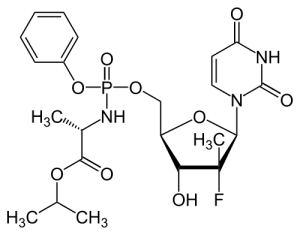

Solvaldi is a member of a class of antiviral drugs called nucleoside analogs. They act as chain terminators and inhibit viral RNA synthesis. When the viral RNA polymerase is copying the viral RNA, to enable the production of more virus particles, it normally uses the pool of ATP, UTP, GTP, and CTP to produce more RNA. When Solvaldi is incorporated into the growing RNA chain by the viral enzyme, no additional triphosphates can be added, because the drug contains a fluorine atom at the 2′-position of the ribose. Its presence inhibits addition of the next nucleoside by the polymerase to the 3′-OH. Viral RNA synthesis therefore stops, and production of virus particles is inhibited. For more information on chain terminators, see my virology lecture on antivirals.
Gilead believes that the price of the drug is fully justified: a spokesperson said “We’re just looking at what we think was a fair price for the value that we’re bringing into the health care system and to the patients.”
It could cost up to $300,000 to treat patients with chronic HCV infection using less effective and more difficult to tolerate regimens. The potential benefit of a cure for patients with liver disease is clear, as the virus is the main reason that nearly 17,000 Americans are waiting for a liver transplant. The need for a well-tolerated, effective regimen is equally critical for people infected with HIV and HCV, because having both infections accelerates liver damage.
Despite these arguments, the high price will be a significant barrier for many, especially those in limited and fixed-budget programs such as Medicare and Medicaid. A panel of experts in San Francisco estimated that switching HCV infected Californians to Sovaldi would raise annual drug expenditures in the state by at least $18 billion.
Gilead has agreed to help U.S. patients pay for Sovaldi if they cannot afford it, or help patients obtain drug coverage. The company also plans to charge substantially less for a course of treatment in India ($2000 for the 12 week course), Pakistan, Egypt ($990 for the 12 week course), and China, where most people infected with HCV live. These deals have prompted some to ask if the US is being forced to subsidize the cost of the drug worldwide. I personally do not object to helping other countries solve their HCV problem.
What is a fair price for a drug that can eliminate HCV infection? Gilead paid more than $11 billion in 2011 to acquire the company that developed Sovaldi, and it is reasonable for them to recoup that investment. Andrew Hill of the Department of Pharmacology and Therapeutics at Liverpool University estimates the manufacturing cost of a 12 week course of treatment with this drug to be $150 to $250 per person. The answer to our fair price question must lie somewhere between these extremes.
There are parallels between Sovaldi (and other new anti-HCV drugs in the pipeline) and the initially expensive antivirals that were introduced ~20 years ago to treat HIV. Anti-retrovirals revolutionized the treatment of a chronic, lethal infection that is major global health problem, and the anti-HCV drugs could have the same effect. But there are also important differences: based on the number of infected individuals, HCV is a much larger public health threat than HIV. Furthermore, the new HCV antivirals can eliminate the virus completely, whereas anti-HIV drugs only suppress virus replication, so they must be taken (and paid for) for life.
At some point in the future competition among pharmaceutical companies and manufacturers of generic drugs should make it possible to treat everyone infected with HCV with affordable, curative antivirals. If the cost and efficiency of diagnosis and drug delivery keeps pace, it might be possible to eradicate HCV. That accomplishment might well be priceless.

Pingback: What price antiviral drugs? | IB Biology | Sco...
Our clinicians have been holding off from treating many patients until these new drugs came along, they term in “warehousing”.
It is interesting to note that for patients with asymptomatic HCV infection, the very long time course of disease progression could make it feasible to “warehouse” until the patent runs out. Wonder if Gilead realised that when they set the price?
“because the drug contains a fluorine molecule in place of the hydroxyl that is normally used to add the next nucleoside.” The fluorine looks to be in the 2′ position not the 3′ position where the next nucleotide would be added.
I wouldn’t be surprised if the next new class of antibiotics (whatever that may be) doesn’t end up being really expensive. That would make it much more likely that resistance is avoided, and provide a return on investment for what is typically a drug taken for only a short duration. Of course, no one will buy it if there are viable alternatives, but the way things are going, there may not be very many before too long. They may not cost $84000, but who knows. If XDR TB or NDM-1 infections start showing up in the States in large numbers who is going to say no to patients?
You are right, the F is at the 2′-position of the ribose. It inhibits addition of the next nucleoside at the 3′-OH. Fixed.
The patent lasts 17 years, I believe – would a physician wait that long to treat? Seems unlikely.
In a way our clinicians are playing a game of “chicken” with the virus as many patients will show only symptoms >20 years after infection.
For our patients the price tag means therapy is unlikely to be sanctioned unless there’s real evidence of clinical need due to liver damage, not quite what was hoped for.
While you’re fixing that section, you might also want to change “fluorine molecule” to “fluorine atom”.
Pingback: A combination pill for hepatitis C
Pingback: The Herculean task of tackling hepatitis C continues | MRC-University of Glasgow Centre for Virus Research science blog
Creative Animdoel offers gene knockout(http://www.creative-animodel.com/Animal-Model-Development/Knockout-Services) products with reasonable price.
list of virology drugs post exposure
pre expousure to virology toxins
list of pre exposure anti hiv medications