Why is the incidence of infection with 2009 H1N1 influenza highest among 5-24 year olds, and lowest in those over 65 years of age? Were the oldsters previously infected with a related influenza virus, or is there another explanation?
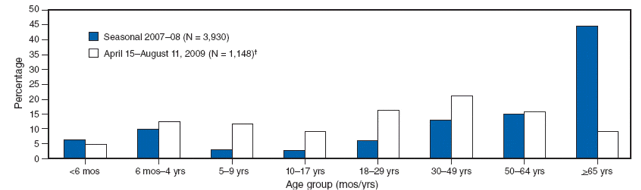
The sera of individuals born in the early part of the 20th century have antibodies that block infection with the 2009 H1N1 virus. We also know that antibodies that prevent infection with recently circulating seasonal H1N1 viruses do not react with pandemic H1N1 strains. These findings may partly explain the lower incidence of influenza this year in individuals greater than 65 years of age (illustrated).
But other factors might also be responsible for safeguarding the older population. Infection of guinea pigs with a 2007 seasonal H1N1 virus confers some protection against infection 3 weeks later with a 2009 H1N1 strain. The animals produced significantly less virus in their respiratory tracts, and transmission of infection to naive animals was impaired. The pre-inoculated animals were also less susceptible to acquiring the 2009 H1N1 virus from another infected guinea pig. Similar results were observed when guinea pigs were first inoculated with a seasonal H3N2 strain and challenged with the pandemic virus. The conclusion is that infection with seasonal H1N1 or H3N2 viruses provides protection against infection with the 2009 H1N1 strain.
The protective effect of the seasonal strains does not appear to involve neutralizing antibodies. Sera from the guinea pigs inoculated with the seasonal H1N1 or H3N2 strains did not contain antibodies with hemagglutination-inhibition (HI) activity against 2009 H1N1 virus. One explanation for the protective response is that the initial infection leads to T cells or antibodies that recognize protein sequences that are shared among different influenza virus strains or even subtypes. This so-called ‘heterosubtypic’ or ‘cross-protective’ immunity is often observed in animal models of influenza.
Can these results extrapolated to humans – in other words, might transmission of the 2009 H1N1 influenza virus in the human population be slowed by previous exposure to seasonal strains? One good example of heterosubtypic immunity after influenza virus infection in humans was provided by analysis of archival records from the 1957 pandemic in Cleveland, Ohio. That outbreak was triggered when the seasonal H1N1 strains were replaced by a novel H2N2 strain. Only 5.6% of the adults who had had influenza before 1957 developed influenza during the pandemic. In contrast, 55.2% of the children who had influenza before 1957 contracted it afterwards. These findings suggest that accumulated heterosubtypic immunity might have an impact on influenza during a pandemic.
These results do not mean that the inactivated seasonal influenza vaccine, containing H1N1 and H3N2 strains, will protect you against the 2009 H1N1 virus: they do not induce heterosubtypic immunity.
The emergence of 2009 H1N1 influenza is a good opportunity to study the effect of heterosubtypic immunity because the strain is antigenically very different from previously circulating seasonal H1N1 and H3N2 strains.
Steel J, Staeheli P, Mubareka S, GarcÃa-Sastre A, Palese P, & Lowen AC (2009). Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. Journal of Virology PMID: 19828604.
Epstein, S. (2006). Prior H1N1 Influenza Infection and Susceptibility of Cleveland Family Study Participants during the H2N2 Pandemic of 1957: An Experiment of Nature The Journal of Infectious Diseases, 193 (1), 49-53 DOI: 10.1086/498980.

As suspected, only the live virus nasal spray is available for h1n1 in my area. They turned me away b/c I was breastfeeding and said I could not have it. My ob said he'd prefer the injection but either one is okay, my child's doctor said wait for the shot, the CDC and WHO say breastfeeding is NOT contraindicated for the nasal spray. I'm confused, why is it not okay for nursing mothers? Can anyone help me please? Odds are I will be bringing this crap home before we can get a shot. It just seemed more like risking my son's life by not getting the vaccine and having it deliver the antibodies into my milk than by getting the cooked version. Can you please help me with this, as I can not decide what to do. Thank you-Erica
Pingback: Tweets that mention Being older is a good defense against 2009 H1N1 influenza virus -- Topsy.com
Pingback: uberVU - social comments
Can you comment this information? – http://www.abovetopsecret.com/forum/thread51545…
http://www.freepatentsonline.com/y2009/0010962….
http://www.youtube.com/watch?v=KmhREAMmfhg&hd=1
Is it known how important T-cell vs antibody response is against influenza? If the H and N molecules change a lot faster than the rest of the virus, it seems like the virus might often mutate so that your neutralizing antibodies don't work, but you could still get over the flu faster because of the CTL response. (Would Th1 response also be important here?) Is this likely an explanation for how some people seem to carry the flu without getting very sick?
I'm curious how this might interact with getting the Flumist instead of the inactivated vaccine. On one hand, my CTLs might suppress the infection too fast because it recognizes the similar protein fragments from previous infections. On the other hand, the live virus can give me an effective CTL response for the next time I'm exposed.
Given that heterosubtypic immunity is not triggered with regular seasonal flu shots, could it be that patients who succumb to severe and fatal Novel A/H1N1 2009 infection have received regular seasonal flu shots over the past few years? I wonder if it is the common denominator for patients in the 31-64 age-range some of whom have had known health risks and others who have not. Certainly patients with known health risks such as asthma or diabetes would be more likely to have received seasonal flu shots annually.
http://public-healthcare-issues.suite101.com/ar…
Then there's the 10 year study of children with asthma (link above) that showed increased hospitalizations for ILI with asthmatic children who had received seasonal flu shots vs. those who had not. Could development of heterosubtypic immunity be relevant in these cases as well?
Wonder if this will be investigated further…
There is no reason why you can't have either influenza vaccine while breastfeeding. The infectious vaccine (Flumist) should not be given during pregnancy; but post-partum administration is fine.
The patent applications discussed in the links you sent relate to producing swine influenza viruses with an alteration in the protein NS1. This was being done to produce an attenuated vaccine. The patent application was submitted around 2004. To believe that this means the current swine flu is 'engineered' in a laboratory is incorrect. Swine influenza viruses have been in pigs since 1918 and there are many of them. The swine virus used in this patent application is very different from the 2009 H1N1 swine-origin influenza virus. I'm frustrated at the rampant misunderstanding; apparently not everyone reads this blog.
Influenza virus-specific CD8 T cells do not prevent infection but are important for recovery from infection. The epitopes are known and are conserved, hence are cross-protective. See Immunology and Cell Biology (2009) 87, 300–308 for a good recent review. These cells might control disease severity in different hosts. Yes, Th1 cells would be important a they drive proliferation of CD8 T cells. I think Flumist would basically mimic a natural infection and hence there won't be issues with too rapid clearance.
It's very possible. There haven't been sufficient studies to know if homotypic immunity conferred by vaccines predisposes to more severe pandemic disease. Check back Friday for a post on this issue stimulated by a recent Lancet article. I'm sure it will be investigated further; although the results are from animals they are compelling.
Pingback: Is yearly influenza vaccination of children a bad idea?
FWIW – Nature 326, 449-450 (8 April 1987) | doi:10.1038/326449c0 “Cause of death of the American Indians” discusses possible swine flu origin influenza epidemic in 1493.
http://www.nature.com/nature/journal/v326/n6112…
Thanks for that letter. Interesting that pigs are discussed as
possible vectors for influenza. It is my understanding that influenza
went into pigs for the first time in 1918, and wasn't known before
that.
Yes, Webster et al say in their review that, “The influenza viruses currently circulating in humans and pigs in North America originated by transmission of all genes from the avian reservoir prior to the 1918 Spanish influenza pandemic.” (http://mmbr.asm.org/cgi/content/abstract/56/1/152 ) But that is different from saying that 1918 was the first time influenza existed in pigs, and they make clear that inferring dates from phylogeny interpreted sequencing is dicey.
Evolutionary theory would not favor the view that swine adapted influenza never existed before then or first reassorted in 1918. This is supported by the observation that influenza B and C viruses do not occur in birds and C viruses are isolated from pigs and dogs, which indicates that these diverged a very long time ago from the primary avian strains. (See: pg 166 on origins.) That indicates that multiple “cycles” of crossover from avians into mammals have occurred in the past, with the crossover viruses adapting to their hosts. It is doubtless the case that most of those crossovers went extinct, thus B and C strains of humans are relics of past events.
So I don't think anyone can say for absolute certain one way or another, only infer from phylogenetic evidence, and negative phylogenetic evidence is weak with influenza because of the high probability that lineages can die out, particularly when there were fewer people and pigs and those populations had poorer interaction networks.
My view is that water birds and other fowl have been infecting pigs with influenza for thousands of years in human communities. The fact that water where waterbirds live can be laden with influenza viruses and that those viruses can remain infectious for long periods in water, dependent on salts, pH and temperature, strongly indicates that the conditions have existed for influenza transmission to pigs anywhere that pigs were kept as livestock in human history. (I believe not keeping pigs as livestock has bearing on the immunological evolution of Native Americans relative to influenza.)
I wish I could find actual titer and CD8 activation data on various vaccines such as Flumist vs injected to compare. I'm probably just not searching properly for it.
Thanks for that letter. Interesting that pigs are discussed as
possible vectors for influenza. It is my understanding that influenza
went into pigs for the first time in 1918, and wasn't known before
that.
Yes, Webster et al say in their review that, “The influenza viruses currently circulating in humans and pigs in North America originated by transmission of all genes from the avian reservoir prior to the 1918 Spanish influenza pandemic.” (http://mmbr.asm.org/cgi/content/abstract/56/1/152 ) But that is different from saying that 1918 was the first time influenza existed in pigs, and they make clear that inferring dates from phylogeny interpreted sequencing is dicey.
Evolutionary theory would not favor the view that swine adapted influenza never existed before then or first reassorted in 1918. This is supported by the observation that influenza B and C viruses do not occur in birds and C viruses are isolated from pigs and dogs, which indicates that these diverged a very long time ago from the primary avian strains. (See: pg 166 on origins.) That indicates that multiple “cycles” of crossover from avians into mammals have occurred in the past, with the crossover viruses adapting to their hosts. It is doubtless the case that most of those crossovers went extinct, thus B and C strains of humans are relics of past events.
So I don't think anyone can say for absolute certain one way or another, only infer from phylogenetic evidence, and negative phylogenetic evidence is weak with influenza because of the high probability that lineages can die out, particularly when there were fewer people and pigs and those populations had poorer interaction networks.
My view is that water birds and other fowl have been infecting pigs with influenza for thousands of years in human communities. The fact that water where waterbirds live can be laden with influenza viruses and that those viruses can remain infectious for long periods in water, dependent on salts, pH and temperature, strongly indicates that the conditions have existed for influenza transmission to pigs anywhere that pigs were kept as livestock in human history. (I believe not keeping pigs as livestock has bearing on the immunological evolution of Native Americans relative to influenza.)
I wish I could find actual titer and CD8 activation data on various vaccines such as Flumist vs injected to compare. I'm probably just not searching properly for it.
Pingback: Protection against 2009 influenza H1N1 by immunization with 1918-like and classical swine viruses