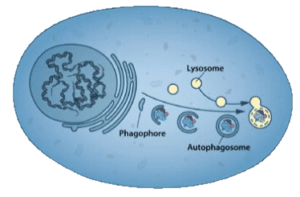

The word autophagy was coined by Christian de Duve in 1963 to describe a process that he and others had previously described: when stressed, cells would sequester portions of the cytoplasm in double-membraned vesicles called autophagosomes. These would then fuse with lysosomes (which de Duve had discovered) and the contents were degraded (illustrated; image credit).
In subsequent years it was suggested that autophagy might have roles in human disease, but little progress was made on understanding how the process worked: how it was triggered, what proteins were involved, and its function in health and pathogenesis.
As a young Assistant Professor at Tokyo University in the early 1990s, Ohsumi found that autophagy occurred in the yeast Saccharomyces cerevisiae. He decided to produce yeast strains lacking the proteases that would be involved in digesting the contents of the autophagic vesicles, with the idea that these vesicles would accumulate under stress (under normal conditions the autophagosome exists for a short period of time, making its study difficult).
When Ohsumi stressed the yeasts lacking the vacuolar (equivalent of mammalian lysosome) proteases, autophagosomes accumulated in the cytoplasm which were readily visible by light microscopy. He used this clear phenotype to isolate yeast mutants that could no longer accumulate such vesicles, and identified 15 genes that are essential for induction of autophagy in eukaryotic cells.
Later Ohsumi elucidated the functions of these genes in autophagy induction, and found homologues in mammalian cells. His work stimulated great interest in autophagy, and it became a highly studied pathway. We now understand that autophagy is not only important for embryogenesis and cell differentiation, but plays roles in neurodegenerative diseases, cancer, and in defenses against bacterial and viral infections.
As might be expected, virus infection is a stress that triggers autophagy, which may impact the outcome of infections: it can have both antiviral functions, and it can also stimulate virus replication. As might be expected for a cellular process that degrades cytosolic contents, autophagy can lead to clearance of viruses. Consequently a number of viral genomes encode proteins that inhibit autophagy, including herpes simplex virus. Degradation of viral proteins by autophagy can also provide peptides for presentation to the cellular adaptive immune system, further enhancing clearance.
Autophagy may also benefit virus replication. For example, non-lytic cell to cell spread of poliovirus depends on release of virus particles from autophagosomes, and autophagy of lipids provides metabolic energy for dengue virus replication.
When autophagy was first described in the 1950s, no one knew its significance. Nevertheless, a number of scientists, including Ohsumi, continued to study autophagy because they were curious. The unexpected result was the elucidation of a pathway that has substantial roles in a variety of human diseases.
The lesson is clear: let scientists pursue their curiosity. It’s fine to target specific research problems, like curing cancer or diabetes, but don’t ignore fundamental research on problems that don’t seem to be directly relevant to human diseases. I’m concerned (as are many others) that the US science establishment is moving away from fundamental research (whose benefits may not be apparent for a long time) to translational research.
How do we explain the trend from fundamental to translational research? I don’t have all the answers, but I think part of the problem is that the US Congress likes to spend taxpayer money on targeted problems, like Alzheimer’s disease. But tell them that you want to study a process in cells that looks interesting, but you are not sure what it means, and you can see their reluctance to support this type of work.
