
A protective variant of the prion protein was discovered in the Fore people of Papua New Guinea. Beginning in the early 1900s, the prion disease kuru spread among Fore women and children as a result of ritual cannibalism of the brains of deceased relatives. When cannibalism stopped in the late 1950s, kuru disappeared.
Survivors of the kuru epidemic are heterozygous for a prion protein gene (prnp) with a unique amino acid change not seen in other populations, a change at position 127 from glycine to valine (G127V). The G127V change was always seen together with methionine at 129. Heterozygosity for M and V at amino acid 129, which is protective against prion disease, is found in humans all over the world.
Transgenic mice were used to determine if the G127V change in prion protein protects against disease. These mice lack the murine prnp gene (which encodes the normal prion protein) and contain a copy of either the wild type human prnp gene, or one with changes at amino acids 127 and 129. The mice were then inoculated intracerebrally with brain extracts from individuals who died of kuru. Mice with wild type human prnp were susceptible to infection. In contrast, transgenic mice heterozygous for the variant prnp (G127M129/V127M129) were completely resistant to infection. The mice were also resistant to infection with prions from cases of another human TSE, Creutzfeldt-Jacob disease.
Prnp transgenic mice were also challenged with variant Creutzfeldt-Jacob disease prions. This novel TSE arose after consumption of beef from animals with the prion disease bovine spongiform encephalopathy (BSE). These mice were susceptible to infection with vCJD prions, not a surprising result given that the Fore people were never exposed to BSE prions. However, mice homogygous for the altered prnp (V127M129/V127M129) were completely resistant to infection with vCJD prions – as resistant as mice with no prnp genes.
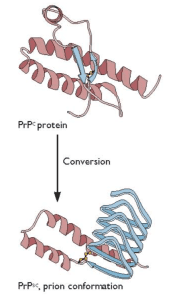
The protective effect of the M129V polymorphism is thought to be a consequence of inhibition of protein-protein interactions during prion propagation (i.e. the conversion of normal prion to pathogenic prion). How the G127V change confers protection is unknown.
These results show that the G127V change confers resistance to kuru and was likely selected as a consequence of the epidemic. If kuru had not been stopped by the abolition of cannibalism, it likely would have been self-limiting, as individuals with resistance to the disease, caused by the G127V change, repopulated the Fore people.

Pingback: Why cannibalism is a bad idea. – Molecular Musings