
When the gut microbiome of mice is altered by treatment with antibiotics, subsequent intranasal infection with influenza A virus leads to reduced antiviral antibody and T-cell responses. The antibiotic treatment does not cause a general immunodeficiency – the mice can respond normally to protein antigens.
The defective immune response to influenza virus in antibiotic treated mice can be rescued by treating the mice with compounds that stimulate the innate immune response – such as lipopolysaccharide, a bacterial product. These compounds rescue the immune defect when administered either intransally or rectally at the time of influenza virus infection. Apparently stimulating the innate immmune response in the gut is sufficient to correct an immune defect in the lung.
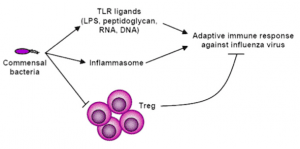
How might gut bacteria be important for immune responses to a lung infection? When influenza virus infects the lung, development of immune defenses depend upon a complex of several proteins called the inflammasome. This structure is needed for the production of cytokines that promote adaptive immune defenses: antibodies and T cells. These cytokines are also needed for the activity of dendritic cells, sentinels that sense a virus infection, and travel to the nearby lymph nodes to inform T cells that there is a problem.
Antibiotic treatment of mice impairs the influenza virus-induced production of inflammasome-dependent cytokines. These results are consistent with the finding that antibiotic-treated mice respond normally to infection with herpes simplex virus type 2 and Legionella pneumophila, two pathogens for which the inflammasome is not required for adaptive immune responses. Furthermore, microbe-mediated inflammasome activation is needed for migration of lung dendritic cells to lymph nodes. In antibiotic treated mice, lung dendritic cells fail to migrate to local lymph nodes. Hence T cells are not informed of the infection, leading to poor antibody and cellular responses.
These findings reveal a link between the gut microbial community and inflammasome-dependent activation of cytokines. How gut bacteria effect this process is not understood. One idea is that bacterial products stimulate white blood cells in the intestine to produce compounds that migrate to the lung and activate the inflammasome.
If you are wondering about the practical consequences of these findings, read the last paragraph of the paper:
Because antibiotic use is prevalent in the treatment of respiratory infections, our results imply a possible deleterious effect of such treatment in initiating proper immune responses to influenza virus. Conversely, it will be important to determine whether probiotic therapy can be explored for immune-stimulating effects during the flu season.
Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, & Iwasaki A (2011). Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America, 108 (13), 5354-9 PMID: 21402903

I wonder if this process could be playing a role in other tissues, such as: nasal/urogenital mucosa, or is it something special to do with the gut and its immune-associated tissue?
That is a great question, one that can easily be investigated. Is there a special gut-lung axis, or does the gut influence extend elsewhere? I’d guess that it would be a broad effect but my knowledge of immune development is lacking.
That is a great question, one that can easily be investigated. Is there a special gut-lung axis, or does the gut influence extend elsewhere? I’d guess that it would be a broad effect but my knowledge of immune development is lacking.
maybe impaired nutrition ?
some components are not being formed
when the bacteria are missing
What an exciting finding to discuss! One of the most novel aspects of this paper is that Ichinohe, T, et al. looked at how commensal bacteria regulate immune responses in other mucosal tissues-besides the gut.  It is important to recall that antibiotics have a range of absorbencies, depending on the antibiotic, route of administration and tissue.  So in this paper, it seems unclear whether the antibiotic usage was directed solely at gut commensal bacteria, or if the antibiotic used targeted all tissue commensal bacteria, thereby affecting the commensal bacteria that line the entire digestive tract.Â
The intriguing aspect of this paper is that it examines how commensal bacteria (regardless of whether they are gut-specific) affect immune responses in the lung, indicating that there is cross-talk between non-mucosal and commensal-laden mucosal tissue immune cells in fighting infections.  That cross-talk is usually in the form of inflammatory cytokines, such as the inflammasome-derived IL-1 and IL-18, discussed in this paper.  These cytokines are important to be produced at the site of infection to warn the rest of the body of infection and to recruit dendritic cells to help clear the infection.
The role of antibiotics and commensal bacteria have been well known in they’re role in regulating immune responses in the gut.  For example, germ-free mice, which lack commensal colonization, lack effective inflammatory Th17 development, and are more susceptible to infection than their wild-type counterparts.  Therefore, as this PNAS paper illustrates, antibiotics tend to decrease inflammatory responses (IL-1, IL-18 production is impaired which is needed to recruit DCs to the site of infection).  Unsurprisingly, when DC migratory function is reduced it leads to an impairment in virus-specific antibody and T cell development, since the adaptive immune response depends on effective DC activation.How commensals regulate the immune system is an interesting field of research.  Some bacterial mediators are well known, usually expressed by pathogenic bacteria (LPS, is an example which potently activates the inflammatory immune response, and why it was able to reverse the effects seen in the presence of antibiotics.) This paper suggests that either a secretory bacterial product is being produced to induce distal immune modulations, or that perhaps immune cells present near commensal bacteria colonies, directly interact with commensal-specific products that then alter immune cell function.  This paper, really highlights the compelling research happening in the immunology field and leaves many exciting questions to further investigate in the future!  If you’re interested in learning and discussing the data behind today’s most recently published findings in the fields of immunology, medicine and human health, I encourage you to check out Escaping Anergy: The Immunology Research Blog  @ http://escapinganergy.blogspot.com/