
Compounds used to treat HIV-1 infection fall into distinct classes: protease inhibitors (Ritonavir, Saquinavir, or Indinavir), nucleoside reverse transcriptase inhibitors (NRTI, AZT, 3TC, Tenofovir, D4T), non-nucleoside reverse transcriptase inhibitors (NNRTI, Efavirenz, Nevirapine), integrase inhibitors (118-D-24), and fusion inhibitors (Maraviroc). None of the HIV-1 protease inhibitors, NNRTI, or integrase inhibitors blocked XMRV replication. Of the NRTIs, only AZT significantly inhibited viral replication. Fusion inhibitors were not examined in this study.
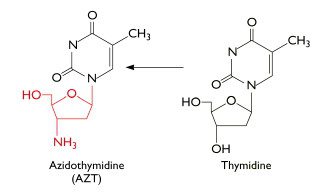
AZT was the ï¬rst drug licensed to treat AIDS. It is phosphorylated to the active form by cellular enzymes. Phosphorylated AZT is an inhibitor of viral reverse transcriptase because it acts as a chain terminator when incorporated into DNA:
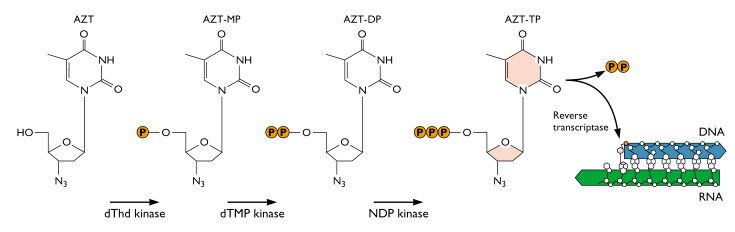

Because AZT has a N3 (azido) group on the ribose instead of a hydrogen, the next base cannot be added to the DNA chain and synthesis stops.
The relative selectivity of this drug depends on the fact that reverse transcription takes place in the cytoplasm, where the drug appears ï¬rst and in the highest concentration. But the presence of AZT monophosphate causes depletion of the intracellular pool of ribosylthymine 5€²-triphosphate (TTP). Therefore AZT has substantial side effects which include muscle wasting, nausea, and severe headaches. AZT treatment can also damage bone marrow, which requires multiple transfusions of red blood cells. The drug was used extensively because there was no alternative until other antivirals were developed.
AZT can be taken orally but it is degraded rapidly by liver enzymes. Patients must take the drug two or three times a day to maintain an effective antiviral concentration. The drug is modestly effective in infected adults, leading to a transient increase in CD4+ T-cell counts.
Much effort has been devoted to discovering alternatives to AZT, and several nucleoside analogs that have therapeutic value, such as 3TC, are available. However 3TC does not inhibit XMRV replication.
It is not known if treatment with AZT will effect either prostate cancer or CFS. If prostate cancer is triggered when XMRV inserts into chromosomal DNA, then the drug will not likely block progression of the disease because the drug does not eliminate infected cells. Whether reduction of viral loads by AZT treatment has a positive therapeutic outcome remains to be determined. Because AZT is approved for use in humans, such studies can proceed immediately, without the need for extensive toxicity studies in animals.
Sakuma R, Sakuma T, Ohmine S, Silverman RH, & Ikeda Y (2009). Xenotropic murine leukemia virus-related virus is susceptible to AZT. Virology PMID: 19959199

Love your blog. Thanks for keeping us up to speed with the latest on XMRV.
You have a typo in the first paragraph–those drugs are surely licensed for use against HIV, not against XMRV.
Will this study be published in a scientific journal and if so, when?
When and who gets to try these drugs?I`m ready before I die so please hurry.
Pamela B.
The study referred to in this post has been published; the reference
is at the bottom of the post. Virology Journal is open access so
anyone can see the article.
Sorry, it looked like a just an abstract. Well, actually, really is just an abstract, but I am assuming that this:
Virology. 2009 Dec 1. [Epub ahead of print]
means it will soon be available in full.
Thanks for your on-line course. I intend to work myself through it as a CFS patient of 39 years, it is important that I understand everything I can about viruses. If XMRV is indeed key, I need to understand as much as I can to work out a treatment protocol.
You are right, of course, scientific illiteracy is dangerous in the extreme.
The full manuscript on this topic is available for downloading at the
Virology Journal website. Of course in my view everyone needs to learn
more about viruses, not just those who suffer from them. That's why I
blog here. You might also want to look at our podcasting efforts at
http://www.twiv.tv where we also cover a lot of virology basics.
Professor R:
I understand that XMRV replicates so slowly that treating it will be diffcult. Does this mean that Isentress or AZT will not work at all or will it just take much longer for a patient to feel better on the drugs?
Professor R:
also, if the virus is just sitting there pumping out proteins(?) could the antiretrovirals still do something?
thank you for your time
Slow replication should not have an impact on the ability of an
antiviral compound to inhibit viral replication. On the contrary, it
might reduce the changes of selecting drug resistant variants.
I think you are asking, if the viral genome is integrated into the
host chromosome, and only viral proteins are being made, will the
antivirals be effective? Since AZT targets reverse transcription, the
drug will not have an impact on particle production – it will only
block new infections. But that approach is highly effective for
treating AIDS. The problem is that the antiretrovirals do not
eliminate cells in which the viral genome has been integrated.
Professor R:
I ask because Dr. Coffin mentioned that slow replication(?) of XMRVwould make treatment problematic because present drugs target replication, as in HIV… So I guess I wanted to know how the differences between XMRV and would impact treatment.
whoops……”the differences between XMRV and HIV would impact treatment.
I'm not sure what Coffin is referring to: if an antiviral blocks viral
replication – meaning the production of new, infectious virus
particles – then it should not matter how quickly or slowly the virus
replicates. Perhaps you can point me to where he said this.
This is part of what he said in his testimony to the CFS Advisory Commity in October, 2009:
“It’s not that this virus has a lower mutation rate than HIV. These viruses probably have all about the same mutation rate. But its suggestive in fact that there are very few cycles of replication that separate the viruses that’s in one person from the virus that’s in another.”
” And in some ways the implications of that are both good and bad news. The bad news is that it suggests the virus is not actively undergoing ongoing replication during the course of infection of a single individual and that would not be good news if one were trying to use antiviral therapy. “
Just for the record. There is no typo in the first paragraph. It says “Of ten licensed compounds evaluated for activity against XMRV, just one, AZT (azidothymidine), was found to inhibit viral replication.”
I think the “licensed” part in there refers to anti viral drugs in general and ten of those have been evaluated for activity against XMRV.
Professor R:
ME/CFS has kindly posted the Dr. Coffin comment. Why did he say that the slow replication will impact treatment? Is CFS caused by replication of XMRV or the proteins it pumps out during latency?
Thank you
Dr. Coffin said that 'The bad news is that it suggests the virus is
not actively undergoing ongoing replication during the course of
infection of a single individual'. Ongoing replication means the
production of new infectious particles, and infection of new cells by
these particles. If, as Dr. Coffin suggests, the virus is not
replicating at all in humans, then antivirals such as AZT will have no
impact. How the virus might cause disease is completely unknown, so
it's not possible to speculate on how this could affect treatment. It
could be, as you suggest, that viral protein production, not
production of virions, is an essential component of disease. If this
is true then AZT will not be beneficial.
From what you know of this virus now, what would to your preliminairy idea be the best management strategy? Immune modulators maybe still in combination with antiretroviral meds?
Professor R:
Once I had my “% specific lysis” tested at VIP Dx. It was abnormal. Does this imply that the virus is replicating?
What exactly is being measured in the diagnostic assay? I'm unable to
connect with VIP Dx to find out.
Dear Dr. Racaniello:
Since XMRV replicates slowly, do you think, if we take AZT or some drug, it will take us a long time to get better? how long do you think it would take? Do you think, from what we know so far, that one drug will be enough?
Love your blog
Hello. Can stopping viral replication with AZT prevent the cancers that XMRV causes such as lymphoma or leukemia ? especially if you had XMRV for a long time…can you still get cancer even on the antiretrovirals? what is your opinion?
thanks!
Thank you so much for this blog. I just signed up for your ongoing posts/blogs.
Given what you know of XMRV, is there anything you can suggest for us *now*, as in over the counter supplements that might inhibit XMRV?
Best,
Rrrr
Is there any possibility that didanosine could be effective against XMRV? It is my understanding that didadnosine, Like AZT, is reported to one of the wider spectrums of activity against retroviruses including Feline Leukemia Virus, Friend Leukemia Virus,Harvey Murine Sarcoma Virus,Murine Lukemia Virus and Symian T Lymphotrophic Virus.
Thanks,
Dan
Dr. Racaniello:
I would really like to know the possible consequences of late vs early treatment! I am late in the game….what are the implications of having a larger XMRV reservoir in my body even on antiretrovirals? Does this mean less immune reconstutition? More cancer risk?
Also, how would one measure immune recovery in CFS? CD4/CD8 ratio? RNase L activity?
Thank you!!!!
Sue
I would not assume that because the virus replicates slowly that
antiviral resolution will take longer. With a slowly replicating virus
it should be possible to significantly suppress viral replication in a
few weeks. Low replication rates should favor the use of one drug. But
the real question is whether there is a reservoir of latently infected
cells, as with HIV. For HIV these are long-lived and harbor the viral
genome. Currently it is possible to remove nearly all replicating HIV
with antiviral treatment, but the viral reservoir still persists and
we don't yet know how to remove it.
It is certainly possible that didanosine might block XMRV replication.
But then again, I am surprised that 3Tc did not, which is chemically
more similar to AZT than didanosine. I'm sure we'll know the answer
shortly.
Greetings,
Somewhat off-topic:
My wife is a long time PWC, which also suffers from severe angioedema. Her angioedema is said to be explained
by her positive autologous test. The autologous test (ASST) is the one in which one's serum is injected back
subcutaneously, looking for an allergic reaction.
It's hard to assume her problems are not related. Can you think of any mechanism by which XMRV
can explain the ASST results?
Much obliged
Ronen
In AIDS, the resting memory CD4 cell reservoir is established very quickly after infection and doesn't appear to be affected by when ART is begun. Of course, we don't know if similar principles will apply for XMRV.
Dear Professor R:
I came across this abstract recently and found it very intriguing, especially in the context of some of your comments on this blog:
VIRAL SEQUENCE INTEGRATION INTO INTRONS OF CHEMOKINE RECEPTOR GENES
Viral DNA sequences are able to integrate into the non-coding DNA sections of the genome of human cells which have been infected, either spontaneously or experimentally. We have made a data-base search for integration events of non-endogenous viruses into the introns of chemokine receptor sequences. A BLAST search of all viral DNA sequences, using the intronic sequences as “Query,†returned several significant alignments. However, due to the high reiteration rate of the non-coding sequences in the human genome, it became necessary to re-examine the individual alignments to verify whether the virus-flanking intronic sequence was really located in a chemokine receptor intron. We found only one unquestionable event of viral insertion of a section of a long terminal repeat of the murine leukemia virus within the first intron of the CC chemokine receptor 7 gene. Possible biological effects of such an insertion are discussed. Further experimental or clinical research could demonstrate the occurrence of other intronic viral insertions in human chemokine receptor genes. Maria Antonietta Panaro, Immunopharmacology and Immunotoxicology, December 2009, Vol. 31, No. 4, Pages 589-594
If XMRV does something similar, i.e. integrates within/nearby chemokine receptor sequences … and thus affects their expression levels, wouldn’t that help explain quite a lot of XMRV pathogenicity in CFS (and indeed autism, which is my primary area of interest), even in the absence of any replication activity? Raised levels of certain chemokine receptors would make one more susceptible to infections by other viruses that are implicated in CFS and autism (and whose raised levels are also implicated in susceptibility to neurological damage/autism symptomatology caused by HIV for example).
I’m aware that this is all very speculative at this point but would appreciate your input.
I have lived with cfs for the past 18 years and have been exposed to comments which were very disturbing from different Doctors over the years. I am so pleased that there seems to be a different outlook on this dibilitating disorder.
I have lived with cfs for the past 18 years and have been exposed to comments which were very disturbing from different Doctors over the years. I am so pleased that there seems to be a different outlook on this dibilitating disorder.
Pingback: Inhibitors of XMRV
Pingback: a follow-up on bleeding for the cause | BioBlog