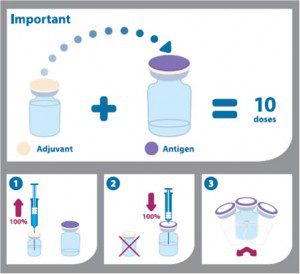

The Canadian vaccine, called Arepanrix H1N1, is supplied in two parts. One contains inactivated H1N1 influenza virus, and the second consists of AS03 adjuvant (DL-a-tocopherol, squalene, polysorbate 80). Before injection the virus and adjuvant are mixed. The vaccine is provided in 10-dose vials and therefore contains thimerosal. More information on the amounts of these components can be found at the Canada Health website (“Product Information Leaflet Arepanrix„¢ H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine”).
Health Canada approved the vaccine based on limited clinical testing, under the provision of an interim order. It is expected that additional safety data will be provided at a later date. The interim order that allows use of Arepanrix H1N1 is based on safety and immunogenicity data on an H5N1 vaccine prepared with AS03 adjuvant in adults and in children, and on 2 separate studies of an H1N1 vaccine prepared with AS03 adjuvant in adults. Canada Health assumes that Arepanrix H1N1 with adjuvant will behave similarly in children and adults as these two tested vaccines, and hence issued the interim order of approval. Let’s examine some of this information.
Adverse reactions: Two studies on H1N1 vaccine were conducted in adults 18-60 years of age. Adverse effects reported included inoculation site pain, redness, swelling, fatigue, headache, arthralgia, myalgia, shivering, and sweating. In general the reactions were reported more frequently in those receiving vaccine with adjuvant. For example, inoculation site pain was reported in 88.9% of 63 individuals who received vaccine with adjuvant, and in 59.1% of 66 who received vaccine without adjuvant. Similar observations were obtained in the second study with 124 individuals.
More extensive studies compared adverse effects of inoculating H5N1 vaccine plus adjuvant in 3,500 adults; the control group was inoculated with buffer. A similar range of symptoms was reported, the most common pain (73%) and muscle aches (33%). Incidence was less in those who received placebo (pain 12%; muscle aches 11.8%). In a separate study, ~300 children 3-5 and 6-9 years of age were given a full or half dose of H5N1 vaccine plus adjuvant. Pain, redness, swelling, fever, drowsiness, irritability, loss of appetite and shivering were more common in those who received the full dose of vaccine. No serious adverse effects caused by the vaccine were recorded.
Immunogenicity: In two separate studies in adults, the H1N1 vaccine lead to seroconversion of 97 and 98.4% of test subjects with or without adjuvant (conversion defined as an HI titer greater than or equal to 1:40). No immunogenicity studies of the H1N1 vaccine in children have been reported. There have been studies in children inoculated with an H5N1 vaccine, but I won’t consider those because the clinical experience is quite different from H1N1 vaccines. The full report can be found here.
Summary: In adults, the H1N1 vaccine approved in Canada induces protective immune responses. The immunogenicity of the H1N1 vaccine in children remains to be determined. In both adults and children, the use of adjuvant leads to more frequent adverse reactions but these are not serious. Consequently Canada Health recommends that children 3-9 years old should be given two half doses of vaccine three weeks apart, and children 10-17 years should receive the full dose (0.5 mL).
Canada has purchased 1.8 million doses of inactivated H1N1 vaccine without adjuvant, but those will not be available until November.

Pingback: Tweets that mention Influenza H1N1 vaccine approved in Canada -- Topsy.com
You say “Canada Health recommends that children 3-9 years old should be given half a dose of vaccine”. I'm currently on Health Can website and it is acutally: “Children from six months to nine years of age should receive the adjuvanted vaccine in two half-doses, administered 21 days apart”
Pingback: uberVU - social comments
What dosage would a toddler who is 2.4 years old be given?
When the article states that the children will be given a half dose of vaccine, does this mean a half dose of the adjuvanted or non-ajuvanted?
Is one dose of adjuvanted enough to provide full protection for an average healthy adult?
Pingback: Influenza H1N1 vaccine approved in Canada | H1N1INFLUENZAVIRUS.US
I think it is half of the adult dose.
see page 5 of the vaccine documentation here:
http://www.gsk.ca/english/docs-pdf/Arepanrix_PI…
For ages 6-35 months the product leaflet reads “Consideration may be given to dosing in accordance with the recommendation in children aged 3-9 years”, which is two half-doses three weeks apart.
Correct, I've fixed the post. Thank you.
“vaccine is provided in 10-dose vials and therefore contains thimerosal”
My children will get this, but I thought bacteriostatics (and therefore multi-use vials) are no longer used in routine childhood vaccination schedules.
If this is speeding up the process I get the benefit of using it in a non-routine situation. But if the provincial health groups are just saving $ they're missing the cost impact of folks not getting vaccinated due to negative publicity about organomercury compounds.
Thimerosal has been removed from all the viral vaccines that children are required to receive; influenza isn't required and (at least in the US) it isn't considered to be part of routine childhood vaccination schedules. Multi-dose vials of the seasonal vaccine also contain thimerosal so it isn't just used in this non-routine situation. I have to agree that given the public's distaste for thimerosal it would be best to eliminate it by discontinuing multi-dose vials of flu vaccine; I believe it would improve immunization coverage.
Yes I can see why those required for school entrance would be extremely sensitive to public concerns. If they can use single doses for the flu programs without sacrificing speed the increased coverage would be desirable. If not it would be good to at least mop up with single doses to expand the coverage.
Thanks again for the background.
“no testing has been performed on arepanrix in pregnant women”
This part has been confusing to some in the Canadian public, as pregnant women are currently amongst the first being encouraged to get the vaccine.
I assume there is data for the pre-existing H5N1 version in pregnant women? I don't see why it would be any less safe during pregnancy than previous flu vaccines?
Government is also saying pregnant women only will have the option to receive non-adjuvanted vaccine when it become available in a couple of weeks. This might lead some to believe the adjuvant poses some risk to pregnant mothers.
From the product leaflet: “No data have been generated in pregnant women with Arepanrixâ„¢ H1N1 nor with the prototype AS03 adjuvanted H5N1 vaccine. Data from vaccinations with seasonal trivalent influenza vaccines in pregnant women do not indicate that adverse foetal and maternal outcomes were attributable to the vaccine.”
I'm not privy to the reason for this policy; however I would guess that because the adjuvants haven't been studied in pregnant women, they are opting for a safe approach i.e. save the non-adjuvanted vaccine for that population only because they are at high risk for complications from H1N1 infection.
I'm a mom in canada with a 7yr old who has ITP (immune thrombocytic purpura). Should I be concerned about him receiving the H1N1 adjuvanted vaccine or should he get the unadjuvanted vaccine that is being recommended for pregnant women?
Sorry, that should be idiopathic thrombocytopenic purpura (ITP).
I'm a little concerned about that Product Leaflet and the fact that it doesn't list all the information as stated by GSK on pg.22
This leaflet is part of a “Package Insert” and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about AREPANRIXâ„¢ H1N1.
What are they NOT telling us?
it probably does..
I've been doing alot of reading lately – and am still concerned about giving my 7 month old the vaccine.
From what I have read – there have not been any tests with the AS03 adjuvant in children under 3 yrs – what has the government used to allow it to be used in infants…are there other trials out there that can give me some peace of mind?
Since the vaccine is meant to be given in 2 doses 21 days apart – I've read it can take up to 38 days for immunity to take effect in most people – isn't this a bit too late?
The unadjuvanted version available shortly for pregnant women…I read that it will contain more thimerosol than the adjuvanted vaccine …is this a better option (if we can get it) for small children?
I don't want to succumb to all the fear-mongering….I just want to keep my child safe and do not want to regret giving her a vaccine that might harm her…I also do not want to see her get ill…I'm just not seeing enough reference being made to the lack of data in small children and wonder why…
Thanks for having this blog …I'll keep reading – and hope to get off the fence real soon!
a half dose would give
half expected side effects ?
half protection (presumably more ?)
Pingback: links for 2009-10-31 « thedysh
I am a nurse. I am 52 years old. I have received the vaccine two days ago and only had pain to the inoculation site and it's gone now. I usually react to all vaccine with muscular pain and temperature and I had no effect of these sorts. I wish it will go for all of you as well as it went for me. I am giving vaccine tomorrow.
I am currently 13 weeks pregnant and I'm a teacher n a public school. I've read that the World Health Organization has recently stated that the adjuvanted vaccine is safe for all pregnant women. It has also been stated on the CBC News last Friday. What is Canada's thought about this?
I've been told by the local clinic that the non-adjuvanted vaccine may not be available for another 2 to 4 weeks.
I have ITP, Spleen removed a decade ago. Could the vac. make me “get the flu” and then how would I fight it, if my system is already comprimised
I have ITP, Spleen removed a decade ago. Could the vac. make me “get the flu” and then how would I fight it, if my system is already comprimised
Half dose for children is indicated to reduce the severity of side effects which were greater than in adults. Two half doses provides for sufficient antigen for a protective response.
Unfortunately there is not a lot of information on this issue. Exacerbation of ITP has been reported after influenza vaccination (inactivated vaccine) and natural infection, but it's quite rare. Why this occurs is not known, but one possibility is that the immune response stimulation by the vaccine is involved. If this is true the adjuvant could make things worse; but remember we don't know why ITP occurs (hence 'idiopathic').
Because the vaccine is inactivated it will not give you 'flu'. Only Flumist (the infectious, inhaled vaccine) can cause a mild infection.
The WHO statement is based on safety testing in non-pregnant adults. More safety testing of AS03 (adjuvant used in Canadian H1N1 vaccine) or MF59 (adjuvant in European vaccine) is needed in pregnant women.
Thank you for replying.
Hi, thank your for this information!! I have a 10 month old infant that had a moderate reaction to Prevnar/Pediacel one month ago. Fever for two days, chills, vomitting 5-6 times, fussiness , lasck of appetite for two days. Had two fits, but was not consistent with febrile seizure. Broke out in a bad case of excema within days of shot. I am worried about further vaccinations, and now we have to make a decision on H1n1. thought about the non -adjuvenated vaccinne but worried about the level of mercury in the non-adjuvenated vaccine. She is still breastfeeding and has no chronic health issues. Any advice? thanks.
You should be able to get non-adjuvanted vaccine in single dose vials
without thimerosal. The pneumococcal vaccines are quite different and
it's impossible to predict whether the influenza vaccine will provoke
a similar reaction. Any egg or other food allergies?
Last year my daughter, who was 2.5 years old at the time, developed Miller-Fisher Syndrome (a variant of Guillain-Barre Syndrome) shortly after recovering from the flu. Would the adjuvant increase her chances of developing this again, given that it is meant to boost the immune response? I don't know if we even have the option of the non-adjuvanted vaccine in AB, it seems that when it becomes available in a couple of days, it will only be for pregnant women.
I know of no associations between adjuvants and GBS. Back in 1976 the
association was with the swine influenza vaccine, and since then only
a variety of infectious agents, not adjuvants, have occurred in
association with GBS. Here in the US, you are asked if you have a
history of GBS before influenza vaccination (at least I was when I
received it at a major medical center), and if the answer is yes you
won't receive the vaccine. In your case the adjuvant is irrelevant;
whoever gives you the vaccine should know her medical history before
administering vaccine.
My 7 year old healthy child had a seizure 3 days after recieving vaccine for h1n1, she had half a dose .I will not be giving her the second dose as doctors in the hospital said not to. I am now terriffied that this will reoccur to my child who has never had any medical issues.
Thanks for the info. Our family physician had suggested asking for the non-adjuvanted vaccine for her (she didn't say why, hence my question), while a nurse who works with the neurologist thought the adjuvanted version would be fine since her previous episode was not triggered by a flu vaccine. I have read that you shouldn't get the vaccine if you've had GBS, and I've also read that you shouldn't get the vaccine only if you've had GBS triggered by the flu vaccine (if it was triggered by an infectious agent, it's fine…) We will definitely mention her history with GBS when we bring her to the vaccination clinic. We just thought we would avoid the ridiculously long line-ups and not bring her in at all if we knew definitively that she was at a high risk of developing GBSs again.
She has not been tested for eggs but has been for Milk as a newborn she had excema after receiving formula. The allergy test for milk was negative, however due to the exgtensive excema the allergist concluded that it was an intolerance. Once I eliminated all dairy from my diet and discontinued supplemenation with formula the excema diappeared and had not returned until the previous shots. Would you suggest that we ask for further testing before giving further vaccines? ps, i understood that single dose viles without thermerisol was not available in Canada. Are you aware of otherwise? I was also told that the non-adjuvanted vaccine did not elicit a favourable immune response and they are recommending the adjuvanted version because of this. and of course i worry about another reaction. What do you think? Any other advice?Thanks again. Stacey.
My 7 year old daughter is in the same situation – developed GBS 2 years ago but not from a vaccination. I have not been able to find anyone who can give me a definitive answer on this – there is a risk of contracting H1N1 and a risk of her receiving the vaccine. I would like to be able to intelligently weigh these risks and make an informed decision as to whether she should be vaccinated but the information I'm getting from the 'experts' seems to be contradictory.
I am the mother of two special needs children and it has been recommended that this group be vaccinated as soon as possbile. We also have a history of auto-immune disease (RA) in the immediate family. I have been reading up on Squalene oil, and have concerns about it as a possible link to autoimmune issues. Your advice on this please?
31 year old relatively healthy male.
Was diagnosed with epilepsy in the late 80s, (grand mal seizures), my last seizure was in 1995. THis past wednesday I had the h1n1 shot at 6pm, at 9pm I was driving and I had a seizure. I dislocated my shoulder, and caused some ligament damage in the other shoulder, my vechicle is a write off, and my license has been suspended for 3mths for medical reasons. This flu shot should come with a warning for those that have experienced past neurological issues. I regret ever taking this piece of crap flu shot!.
If an adult receives HINI vaccination on Day 1, starts to have flu like symptoms, e.g Cough on Day 2, fever Day3, starts Tamiflu on Day 2 evening….How are the immunologic pathways are hindered because of Tamiflu.
Does this patient deserves a repeat HINI vaccination OR should have titers done for the antibodies.
There was no Naso-phyrangeal swabs done for the HINI diagnosis.
SO frustrating isn't it? It is tough to make an informed decision when, despite your best efforts, it is so hard to get informed. With some reservation, we went ahead and got our daughter vaccinated 3 days ago. Other than a sore arm, she seems to be fine. We will watch her very closely and (knock on wood) hope that she does not develop any adverse reactions. Considering some of the risks – there have been kids at her school sent home as “probable” H1N1 cases who have received Tamiflu; she has enlarged tonsils which could impede her ability to breathe should she develop flu-related respiratory issues and we also have a baby… these risks combined with the fact that most of the professional advice we have been given does lean towards this being safe, led us to our decision. I guess either with or without the vaccination, we would be worried! Good luck with your decision… and I hope your daughter fares well either way.
According to CDC, if anyone has had a history of developing Guillain-Barre within six weeks of being immunized, they should not receive the vaccine. If the syndrome did not develop in response to immunization there is no contraindication to receiving the vaccine.
Your child might have had the seizure whether or not she had received the vaccine. The decision not to reimmunize is appropriate and would have been made even without prior history of seizure. Please make sure your child was worked up by physicians after the seizure.
Just so you know, it's the Canadian, not the US vaccine, that contains squalene. There is no scientific evidence that links squalene to autoimmune issues. The information linking squalene to autoimmune disease comes from non-scientific websites which pass on rumor and incorrect information.
Tamiflu does not completely block viral replication. Therefore an immune response will still ensue. The respiratory disease that began on day 1 might not have been influenza. Furthermore, day 1 after immunization is too soon to expect protection from the vaccine – that requires 2-3 weeks. Re-immunization should not be needed.
Dear Prof Racaniello, I think you're doing a great job fielding the questions on this topic. Could I ask you for links or references to the H1N1 clinical trial data? I have a kind of influenza 101 lecture coming up, and I would like to give accurate up to date information on vaccination.
Of course, I could cite your blog as my source, but if I want to encourage the students to dig deeper and check the scientific literature, then I also have to do it myself.
DMc
Here are two published studies: N Engl J Med. 2009 Sep 10. [Epub ahead
of print] Response after One Dose of a Monovalent Influenza A (H1N1)
2009 Vaccine — Preliminary Report. AND: N Engl J Med. 2009 Sep 10.
[Epub ahead of print] Trial of Influenza A (H1N1) 2009 Monovalent
MF59-Adjuvanted Vaccine — Preliminary Report. Here is the trial of
the Chinese vaccine: N Engl J Med. 2009 Oct 21. [Epub ahead of print].
A Novel Influenza A (H1N1) Vaccine in Various Age Groups.
Thanks!