The plaque assay is a terrific method for determining virus titers, but it doesn’t work for all viruses. Fortunately there are several alternative methods available, including the end-point dilution assay.
The end-point dilution assay was used to measure virus titer before the development of the plaque assay, and is still used for viruses that do not form plaques. Serial dilutions of a virus stock are prepared and inoculated onto replicate cell cultures, often in multi-well formats (e.g. 96 well plastic plates). The number of cell cultures that are infected is then determined for each virus dilution, usually by looking for cytopathic effect.
In this example of an end-point dilution assay, 10 monolayer cell cultures were infected with each virus dilution. After an incubation period, plates that displayed cytopathic effects were scored with a +. At high dilutions, none of the cell cultures are infected because no particles are present. At low dilutions, every cell culture is infected. Half of the cell cultures showed cytopathic effects at the 10-5 dilution. This is the end point: the dilution of virus at which 50% of the cell cultures are infected. This number can be calculated from the data and expressed as 50% infectious dose (ID50) per milliliter. The virus stock in this example contains 105 ID50 per ml.
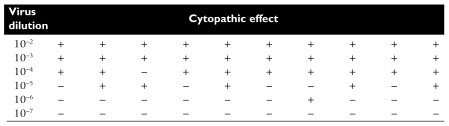

In real life, the 50% end point does not usually fall exactly on a dilution as shown in the example. Therefore statistical procedures are used to calculate the end point of the titration.
End-point dilution methods can also be used to determine the virulence of a virus in animals. The same approach is used: serial dilutions of viruses are made and inoculated into multiple test animals. Infection of the animal can be determined by death or clinical symptoms such as fever, weight loss, or paralysis. The results are expressed as 50% lethal dose (LD50) per ml or 50% paralytic dose (PD50) per ml when lethality or paralysis are used as end points.
The following example illustrates the use of end point dilution to measure the lethality of poliovirus in mice. Eight mice were inoculated per virus dilution, and the end point was death. The statistical method of Reed and Muench was used to determine the 50% end point. In this method, the results are pooled, and the mortality at each dilution is calculated. The 50% end point, which falls between the fifth and sixth dilutions, is calculated to be 10-6.5. Therefore the virus sample contains 106.5 LD50 units.
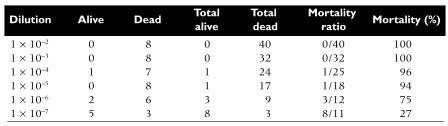

Reed, L.J., & Muench, H. (1938). A simple method of estimating fifty percent endpoints. Am. J. Hygiene, 27, 493-497

Dear sir.
Im VietNamese. I am studying about Varicella virus. I want to determine the titer of virus in vaccine but
I face a problem when choose the media diluent virus and the media for overlay. Can you help me? My email:thuyniheje@yahoo.com. Thank you.
My colleague Saul Silverstein, who worked on varicella virus sent me the following: To titrate virus dilute in PBS containing 2% calf serum and overlay a cell monolayer with a minimal volume of liquid (100ul for a 3.5 cm dish), incubate at 37 and gently tap the sides of the dish even 15 min for 1 hr. After 1hr remove virus and add 3mls of medium with serum and incubate. You should see plaques developing in a few days. You can add 0.1% pooled human gamma globulin to reduce plaque spread. Remove medium, fix with methanol and stain with crystal violet.
great job. Would you please tell us about Karber’s method.?
Thank you very much. But I think the medium with serum that add after remove virus and incubate is very imfortant. Can you tell me which is medium? MEM or other? Thank you.
I am in Viet Nam. I know the Reed and Muench. If you want to know it, I can give to you. Contact me: thuyniheje@yahoo.com.
Hello,
I am making TCID 50, what does it mean, make serial dilutions from virus stock, is it virus supernatant from third amplification step, or purified virus recovered from supernatant by ultracentrifugation. Purified virus is stored in storage medium containing glycerol, can this somehow affect the assay. the protocol i’m using requires me to take 200ul of virus (not sure, the supernatant or the purified virus), if its purified virus, its too much, my cells will die in 2 days, can i some how minimize, the volume?
Thanks
hello
i am here in pakistan a phd student. i need original paper of reed and Reed L.J. & Muench M. (1938) A simple method of estimatingfifty percent end points. American Journal of Hygiene
Reed L.J. & Muench M. (1938) A simple method of estimating
fifty percent end points. American Journal of Hygiene
27, 493–497. for calculation of LD 50.493–497. for calculation of LD 50.
KIND REGADS
What is the equation 4 TCID50 ? How can i explain it to my students ?
Hi friends!
Read the  following. Really helpful in understanding TCID50. Further, I send my new formula for the publication and will upload after acceptance.
http://whqlibdoc.who.int/monograph/WHO_MONO_23_(3ed)_appendices.pdf
M. A. Ramakrishnan, Senior Scientist, Indian Veterinary Research Institute, Mukteswar, IndiaÂ
maramakrishnan@gmail:disqus .com
Hi Dr. Racaniello,
I was reading a paper where they inactivated A/PC using gamma radiation. When they immunized mice in their study they claim that they used doses of 3.2 x 10^6 PFU equivalents. Is the term “PFU equivalent” widely used and what does it actually mean? Would you be able to explain the process of quantifying inactivated virus? Is there a way to use something like a TCID50 equivalent?
Thanks for your help and time!
Nisha
“The 50% end point, which falls between the fifth and sixth dilutions, is calculated to be 10-6.5.” (from the last paragraph)
isn’t it 6th and 7th dilution?
Hello Prof!
I’m a master student working on viroid. Could I use this method for viroid?
Hello Sir,
I am doing PhD in Indian Veterinary Research Institute on Foot-and-Mouth disease virus. Can u please send me the original paper by Reed and Muench on TCID50. I’ll be highly obliged. my email id is sonalikam1@gmail.comReed, L.J., & Muench, H. (1938). A simple method of estimating fifty percent endpoints.Am. J. Hygiene, 27, 493-497Â
Hi,
 I would like to know weather TCID50 can be used to evaluate antiviral activity of plants against Measle , Mumps ,Rubella viruses.
what do you mean by 100 TCID5 0 ?
Dear Dr Vincent,
                  Please send me the original Reed and Muench paper on TCID50. Im a virology student and i think this paper would be the ideal way for me to understand the basics. Thankyou
rohan.vn@hotmail.com
can anyone send me the paper please?
zcbec03 @ucl.ac.uk                 thank you! 🙂
Can anyone send me the original paper of Reed L.J. & Muench M. (1938) A simple method of estimating fifty percent end points. I am looking for the paper by Reed and Muench on the internet and our school library but couldn’t find it. My email is hz0004@uah.edu. Appreciate for your help
Dear Sir.
I have a question about MOI. Some paper says that ‘PK15 cells were infected with PCV2(virus) at an MOI of 1 TCID50 per cell’. I don’t know what’s mean ‘per cell’. Could you please explain about that. Thank you
Hi,
Sir am a Bachelors student of 3rd Semester from Pakistan…I want to know that Does the use of Different virus inocculum in different dilutions effects the final Titer of virus harvest….? Need help on that…And if it does than how and why? here is my email id
syed.zawar01@gmail.com
Will be thankful 🙂
Regards
maramakrishnan@gmail.com
Hi all, as many of you have found, the Reed-Muench paper is not open access and very difficult to track down. However, the WHO Monograph 23 “Laboratory Techniques in Rabies” has detailed descriptions of both the Spearman-Karber and Reed-Muench 50% endpoint calculation methods.
Brett Lindenbach from Yale has a useful Excel spreadsheet implementation of the Reed-Muench method. However I think the latest version 3 has a bug. Do be responsible for checking spreadsheet formulas yourself if you use it and validate that the results are correct (e.g. compare the spreadsheet and manual calculations with a few different data sets first before you start to rely on the spreadsheet). http://lindenbachlab.org/Reed&Muench-v3.xlsx
Oops forgot to provide the link to the WHO monograph, it is http://whqlibdoc.who.int/monograph/WHO_MONO_23_(3ed)_appendices.pdf
It turns out that both the 2008 version 2 and 2011 version 3 have different bugs, and the WHO monograph also has a small but confusing error. Please see my blog post. http://xenobiologista.com/2014/05/tcid50-dont-believe-you-read-everything-on-the-internet/
The original Reed and Muench paper is very nice since it’s from the dinosaur era before computers so they show graphically how the calculation was derived. Sorry I am not able to share it as I obtained it via institutional access.
Dear Sir,
I want to perform a TCID50 for different commercial lyophilized vaccines as a project. I can understand all the procedure but there is only one confusion. How to dilute the lyophilized vaccines to make a 1:10 dilution. I have seen a video on youtube stating that make 1:100 dilution of stock virus then dilute it 10 folds but i am not satisfied with logic. can u please help me out to about how to diluate a lyophilized virus for making a 10 fold serial dilution.
Dear Sir,
I want to perform a TCID50 for different commercial lyophilized vaccines as a project. I can understand all the procedure but there is only one confusion. How to dilute the lyophilized vaccines to make a 1:10 dilution. I have seen a video on youtube stating that make 1:100 dilution of stock virus then dilute it 10 folds but i am not satisfied with logic. can u please help me out to about how to diluate a lyophilized virus for making a 10 fold serial dilution.
If it forms plaques you can try to isolate them. It’s works fine and it’s very easy for some viruses.
More help: lauretti_2000@yahoo.com
Thanks to Dr. Rama for his formula.
One thing I did not understand – if the endpoint test is used for viruses that do not form plaques, how could it work?
I assume viruses that do not form plaque lack a cytopathic effect. I also assume that I’m probably wrong about that, because if they did, I assume the endpoint dilution assay wouldn’t have worked 😛
So how can cytopathic viruses not form plaques? Is it that in the endpoint assay we use cells that does not stick to the plate (such as hematopoietic cells)?
hello sir, i m a bachelor student n wondering the meaning of 19 LD50/0.03 ml of rabies vaccine..i understand the meaning of LD50 but what exactly is that 19 value?? looking for your help..Thank you!
Dear Sirs/Colleague and friends
I have published two simple formula for virus tiration in world journal of virology. one is
Virus titre = (total number of animals died/number of animals inoculated per dilution) + 0.5.
Answer for the above table is = (48/8) + 0.5 = 6.5 (note that you have to include death rate for first dilution also)
This formula will yield similar result as that of Spearman-Karber method. The article can be found at
http://www.wjgnet.com/esps/ArticleInPressDetail.aspx?id=24116
Thanks
M. A. Ramakrishnan
Dear Vincect,
If the virus formed plaques in cell culture, do you think the plaque assay is more robust than the endpoint assay to determine virus titer? Thanks!
just another question, is it correct to assess the tcid50 in the same cell line where the virus was produced?