
So far we have three antiviral drugs against influenza viruses. Most H3N2 strains are resistant to rimantidine, which targets the viral M2 ion channel, although it is still effective against H1N1 strains. There are two neuraminidase (NA) inhibitors: oseltamivir (Tamiflu) and zanamavir (Relenza). The latter is effective against H1N1 and H3N2 strains, but widespread resistance of H1N1 strains to oseltamivir has emerged. Clearly a deeper anti-influenza pipeline is needed.
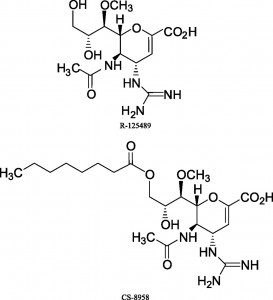
The news is good: there are a number of promising new NA inhibitors in development. One is CS-8958, a modified version of R-125489 produced by Daiichi Sankyo Co. by adding an acyl chain to the 3-O position. The two drugs were tested in NA inhibition assays against a wide variety of H1N1, H3N2, and influenza B viruses. Importantly, CS-8958 was also found to inhibit NA activity of oseltamivir resistant H1N1, H3N2 and B patient isolates. CS-8958 also significantly improved survival of mice infected with a mouse-adapted H1N1 strain. Interestingly, CS-8958 protected mice when administered up to 10 days before infection. Zanamavir was protective when given to mice no earlier than 1 day before infection. These results may not translate to humans, but in the best of circumstances, CS-8958 might have prophylactic value in treating influenza. This property is useful for controlling outbreaks in communities and institutions.
Daiichi Sankyo Co. started a Phase III trial for adults and a pediatric Phase II/III trial with CS-8958 in November 2008. The double-blind study is meant to confirm that a single inhaled dose of CS-8958 is as effective as 75 mg of oseltamivir administered orally twice daily for 5 consecutive days.
Another promising NA inhibitor is A-322278, a modified version of A315675, a pyrrolidine-based compound developed by Abbott Laboratories. A315675 has been shown to be as active against H1N1 and H3N2 strains as zanamavir and oseltamivir. In the study cited below, the effect of the drug on mortality rates, mean weight loss, mean days of death, and lung viral titers was assessed. A-322278 was shown to protect mice against challenge with both wild type H1N1 virus and the oseltamivir resistant NA mutant H274Y.
Other influenza antivirals in development include BioCryst Pharmaceutical’s NA inhibitor, Peramivir, an intravenous/intramuscularly administered drug in Phase 3 development; T-705, an oral RNA polymerase inhibitor from Toyama Chemical, in Phase 2, and DAS181, an inhaled drug from Nexbio, which is in Phase 1. DAS181, or Fludase, is unique in that it incorporates a sialidase that removes sialic acids from mucosal membranes, thereby preventing viral attachment via the HA glycoprotein.
The fact that some of the new inhibitors are not affected by the amino acid changes that lead to oseltamivir and zanamavir failure is encouraging. If the antivirals fare well in clinical trials, it will be possible to devise combinations of the drugs to better cope with emerging resistance.
Yamashita, M., Tomozawa, T., Kakuta, M., Tokumitsu, A., Nasu, H., & Kubo, S. (2008). CS-8958, a Prodrug of the New Neuraminidase Inhibitor R-125489, Shows Long-Acting Anti-Influenza Virus Activity Antimicrobial Agents and Chemotherapy, 53 (1), 186-192 DOI: 10.1128/AAC.00333-08
Baz, M., Abed, Y., Nehme, B., & Boivin, G. (2008). Activity of the Oral Neuraminidase Inhibitor A-322278 against the Oseltamivir-Resistant H274Y (A/H1N1) Influenza Virus Mutant in Mice Antimicrobial Agents and Chemotherapy, 53 (2), 791-793 DOI: 10.1128/AAC.01276-08

Sir its really a nice article,it is very necessary to design the new antivirals as strains of influenza virus are showing the resistancy for existing drugs.I heard that,Tamiflu & Relenza manufactured by Glaxo Smith Klein & Roche Co. are showing side effect in patients. Those including committing to suicide along with some mental disability. Sir will you give explanation on the same.
These effects can observe only in patients, but not in normal individuals (if they also subjected with above mentioned drugs.)So the presence of virus along with the drug is very necessary to induce such kind of mental disturbance.It is a outcome of virus – drug interaction.
Bacterial involvement and high immune response in making the Spanish flu more deadly are acceptable. There might be some genetic evidences from virus for this devastating outbreak. Spanish flu may be genetically more diverged than the existing strains,which made it highly virulent one.what is your opinion on genetic configuration of Spanish influenza as comparable to existing influenza virus strains.
What about Peramivir developed by Biocryst?
Very encouraging PII results… with 100x drug concentration vs Tamiflu. It's in P III trials in Japan done by Shionogi.
Historical Facts Believed To Be Associated With Influenza:
The last influenza pandemic that occurred in the United States was nearly 100 years ago, and this deadly outbreak resulted in about 50 million deaths worldwide. The pandemic that occurred before this one happened about thirty years before the 1918 flu. Influenza epidemics typically occur about every 8 months or so. Influenza is caused by a virus, which is a parasite that needs a host to survive and reproduce.
It was called the Spanish Flu because the first human case was identified there. The pandemic ended up killing more than those that died during WWI. Understandably there was panic among people worldwide, as influenza was not discovered until 1933, so the mystery was rather frightening of what was happening.
Those who survived have allowed others to obtain antibodies from them to develop other antibodies for future viral outbreaks that may occur with this type of virus. This last influenza pandemic also allowed others to obtain this virus from those who died as a result to facilitate effective treatments and vaccines for viral outbreaks that may happen in the future as well.
The virus responsible for the 1918 pandemic was an avian influenza. Nearly 700,000 people in the U.S. died as the result of the Spanish Flu- and those that did die was due often to a bacterial pneumonia that followed the viral invasion and damage. Ultimately, this pandemic killed nearly 3 percent of humans infected. Normally, an influenza strain may kill less than one percent of those infected. The Spanish Flu caused an unusually severe immune response in the human host which made it very deadly due to overkill of the cells of this host.
The influenza viruses are categorized as A, B, and C. The Influenza A virus is the one that historically has caused pandemics that have developed-, such as the Spanish Flu Pandemic. The other influenza pandemics primarily have occurred in countries in Asia.
With influenza, it is understood that the disease influenza is a disease caused by a RNA virus that can infect both mammals and birds. In fact, this particular virus can mutate to where it can be shared between the two life forms and multiply within each one of them. Unlike coryza, influenza expresses symptoms more severely, and usually lasts two weeks until one recovers who has the flu. Influenza, however, poses a danger to some with compromised immune systems, such as the chronically ill, so the recommendation is greater in such populations, along with women who may be pregnant during the flu season, residents of nursing homes or chronic care facilities. Health care personnel are encouraged to get the flu vaccine as well. Such populations allow influenza to progress to deadly pneumonia.
Symptoms of influenza usually start to express themselves symptomatically about two days or so after being infected with the virus. Over 10 percent of the population is infected with this virus every year- resulting in about 200,000 hospitalizations and nearly 40,000 deaths. This season’s first influenza case was identified in Delaware in November of 2008, and it was a type B influenza strain.
The flu vaccination is trivalent- meaning it contains three viral strains of suspected viruses for flu outbreaks during a particular winter season, as determined by the World Health Organization, as well as the Centers for Disease Control, and other organizations. Unfortunately, the influenza vaccine administered last flu season was largely ineffective due to unsuspected strains of the virus infecting others, although about 140 million injections of this vaccine were administered.
After giving the vaccination dose to one, it takes about 10 days for that person to build up the immunity for the disease of influenza. The months of October to December are recommended to receive this vaccine. And the vaccine is about 50 percent effective in offering protection from influenza, according to others. Vaccines are a catalyst for antibody production in humans, which protect them against the virus. Influenza vaccines can be given by injection or nasally.
Anti-virals, on the other hand, decrease greatly the ability for viruses to reproduce once established in a human.
The Avian influenza that many have heard of is potentially the next flu pandemic- as humans have no immunity to what is called the H5N1 virus- on of about 1 strains of avian Influenza. For an Influenza pandemic to occur, which means a global disease existence, the virus must emerge from another species to humans without immunity, as well as the ability to make more humans ill than normal. Also, the virus must be highly contagious for a pandemic to occur. The H5N1 virus appears to replicate in the human GI tract and also has a longer incubation period in humans, one to two weeks, compared with other influenza strains. The H5N1 Avian influenza virus seems to have become progressively more pathogenic in the past decade, according to others.
With the Avian Influenza existing with the H5N1 strain, millions of birds have been slaughtered due to the danger and unpredictability of this strain. The first human case infected with this strain occurred in China in 1997. The first human avian flu case outside of China was identified in 2003 in the Netherlands. The first recorded incidence of human-to-human transmission of the H5N1 virus was in Thailand in 2004. In 2006, it was discovered that the H5N1 had split into two separate strains. There have been outbreaks of Avian flu in about 15 countries in the world so far- with Indonesia being the worst. Migratory birds spread this influenza virus between continents.
The pathogenic strength of the H5N1 strain varies due to constant re-assortment or switching of genetic material between the viruses- essentially creating a hybrid of what it was before this occurs. So far, about 300 people worldwide have been infected with this strain- and about half have died from the infection. Vaccinations are being developed and reformulated constantly at this time due to the pandemic threat of the H5N1 Influenza virus.
Yet, the normal flu season that is now occurring was supplied with 150 million vaccines in the United States. However, some studies have shown that this vaccine is rather ineffective based on incidences of the acquisition of the influenza virus by others anyway.
The influenza season peaks between the months of January and March. The vaccine for this influenza season is manufactured by 6 different companies. Yet the strains chosen are speculated influenza viruses, as this does not eliminate the chance of a new and dominant influenza viral strain that possibly could cause a pandemic. It takes manufacturers about 6 months to make and formulate the influenza vaccination. There is a vaccine for this illness that is produced every year according to which type of virus may be prevalent during a particular flu season. If influenza occurs in a human host, the results may be the patient acquiring pneumonia or meningitis.
The presence of influenza can be widespread in certain states, yet not others. The vaccination is recommended to be administered to those who are at high risk, such as the chronically ill. Also, it is recommended that those under 18 years of age get the vaccine, as well as those people over the age of 50. Furthermore, those people who regularly take aspirin should receive the vaccine, as the influenza disease can become a catalyst for what is called Reye’s Syndrome. Pregnant women should receive the vaccine as well- as there are many other vaccines available to fortunately prevent other diseases, perhaps.
http://www.cdc.gov/flu/weekly/
Dan Abshear
Thanks for reminding me about Peramivir. We are actually talking about
it today on TWiV.
Is there a link to connect? thank you
Correction: peramivir (i.v.) is currently being tested in the outpatient setting in a Phase 3 pivotal trial in Japan with BioCryst's partner, Shionogi
It was called spanish flu because Spain was neutral in WWI and were therefore not censoring their media. The first cases were at Camp Funston in the United States.
Pingback: TWiV 18: Can a virus make you fat?
If the H274Y mutation confers resistance to peramivir as it does to tamiflu what is the point of it?
Several points:
1. Peramivir has superior plasma half life, so single doses can be
used, and it is effective prophylactically.
2. Peramivir is still effective against H3N2 strains.
3. Next season's H1N1 strain might not be resistant to Peramivir.
H274Y is now fixed in H1N1 throughout the northern hemisphere. Evolving away from H274Y at this time will not be easy, and implications for H5N1 are quite real.
Thanks for the comment, Henry. What appears difficult to us may be
facile for a virus. After working on viruses for over 30 years, one
thing I have learned is never to make assumptions about them. Yes,
H274Y is here now, but other selection pressures, which we do not
understand, may select for it loss.
But the real answer, I suppose, is that peramivir was developed before
H274Y emerged, and now the company needs to make a difficult decision.
And it still works against H3N2 strains.
Vincent, I have been studying viruses for 35 years, but most of the understanding on influenza evolution has happened in the past few years due to the dramatic increase in sequence data.
As a result, changes are much more predictable and calling the increase of H274Y to 100% of H1N1 was a pretty easy call. It was due to genetic hitch-hiking (which involved multiple introductions onto multiple H1N1 clades and sub-clades), so eliminating H274Y at this time will be difficult, especially because osletamivir influence on H1N1 frequency is not a factor at this stage.
However, oseltamivir may play a role in moving H274Y from H1N1 to H5N1, which is one of the reasons Roche took such a big hit (they also argued that oseltamivir was still effective against H3N2 and H5N1).
Any antiviral that is inhibited by H274Y will have problems.
Vincent,
My post regarding peramivir was based on the abstracts of two articles authored by Baz M, Abed Y, Boivin G
“The peramivir-selected variant had a H274Y mutation in the neuraminidase (NA) gene conferring resistance to peramivir and oseltamivir” Antiviral Research 2007; 74:159-62 and
“the H274Y mutation conferred resistance to oseltamivir (754-fold increase) and peramivir (260-fold increase) in the N1 subtype only…..It suggests that certain drug-resistant NA mutants may still be virulent although additional studies using clinical isolates are needed to confirm our results.”Antivir Ther. 2006;11(8):971-6
However I am forced to consider that at concentrations being trialled (600mg) saying the H274Y mutation is resistant to peramivir is no longer true. (see page #7 of http://www.corporate-ir.net/ireye/confLobby.zht…)
It is not my intention to spread misinformation or generate discussion on mute points, and so I would be interested in your interpretation.
Is the H274Y mutation significant if peramivir can be used safely at the high concentration?
Avian flu or bird flu is a contagious disease of birds caused by influenza A viruses. All birds are susceptible to avian flu infection, yet wild bird species carry 25 sub-types (16 HA or hemagluttinin and 9 NA or neuraminadase) of influenza viruses without being harmed by them. As such, wild birds are considered the natural reservoir of influenza viruses.
John, note the CDC release. Based on this could Peramivir work against the Tami resistant flu with the higher dose?
Note that Peramivir seems 5-10x more effective against swine flu.
http://www.cdc.gov/mmwr/pdf/wk/mm58d0428.pdf
Until swine flu meets oseltamivir-resistant A(H1N1) influenza.
http://dx.doi.org/10.1016/j.antiviral.2009.03.003
yes but can Peramivir's dose overcome their resistance (they are not totally resistant)
I guess we'll know when Shionogi release the data from phase II and III iv peramivir trials recently completed in Japan. Assuming at least some of the trial subjects had Tamiflu resistant H1N1 (H274Y) the viral titre data will give a strong indication of effectiveness, especially if even one of the subjects had a higher viral load after treatment.
I suppose they could always trial 1200mg after that.
I couldn't help notice things still aren't serious enough for the CDC to look beyond the boarders of Northern America for a preferred solution. Why mention Abbott's A-315675, which is only pre-clinical, and ignore Sankyo's CS-8958 currently in phase III trials in Japan? Bizarre.
Based on the results above the Peramivir vs placebo,IM, trial(P2) in the USA could be iffy depending on the % of participants with the Tami resistant strain.
In Japan the control is Tamiflu with a “non inferior” objective” which should be met leading to approval.
CS-8958 will probably beat Tami in Japan also leading to approval.
I suspect both will be stockpiled in Asia but am unsure how HHS will treat these results in USA.
Any thoughts?
Based on the results above the Peramivir vs placebo,IM, trial(P2) in the USA could be iffy depending on the % of participants with the Tami resistant strain.
New X-ray structures from influenza polymerase (cap-binding domain of PB2 in 2008, endonuclease of PA in 2009) should now open the way for targeting viral RNA replication and transcription processes by developing inhibitors of cap-snatching. Previously Roche worked on the polymerase as at target, but gave up in the absence of structural information. So hopefully this can now be worked upon more productively.
Jake.
can I get synthetic step mechanism??
Joe,
Finally found the trial result data regarding peramivir.
It's tucked away in the Peramivir EUA, Fact Sheet for HCP published a few days ago. I'd like your opinion on the FDA playing hard ball with GSK and not persuading GSK to produce iv zanamivir (a special patent extension for iv zanamivir to make it worth GSK's while to fund trials- after all GSK got screwed over by the FDA and Tamiflu fast-tracking).
“At the onset of the current H1N1 outbreak, FDA contacted GSK about the appropriate regulatory mechanism to provide nebulized zanamivir for U.S. patients on a compassionate-use basis. GSK determined the most expedient approach is through an emergency IND, in which the individual-treating clinician acts as the principal investigator for a single patient. GSK has documentation based on limited data on how to treat. However, the supply of nebulized zanamivir is extremely limited. It was manufactured when GSK was actively developing the IV formulation. GSK feels it should be available worldwide for compassionate-use provided that appropriate regulatory mechanisms are in place. The remaining clinical trial material has been earmarked for compassionate-use. GSK has no intention to restart the manufacturing of zanamivir solution.”
“Following its meeting with FDA, and after reviewing the recent FDA draft guidance, specifically the guidance on clinical trial design in severe hospitalized patients, GSK sees no feasible path to registration; therefore IV-zanamivir remains on hold. GSK will consider a clinical development proposal from a third party for influenza antiviral drug development if it includes a clinical development plan that is reasonable, feasible, scientifically robust, and likely to lead to registration and approval. If the clinical development plan does not meet these criteria, GSK would not support a trial, execute the trial, or provide the drug. However, GSK would not prohibit a third party from sourcing the product from another licensed manufacturer.”
http://www.hhs.gov/aspr/conferences/nbsb/nbsb-h…
What a poor second choice iv peramivir is,
“”In another phase 2 trial (Trial 212) of intramuscular Peramivir 600 mg compared to placebo in patients with acute uncomplicated influenza, a statistically significant treatment effect was not observed for the primary endpoint (time to alleviation of symptoms) or secondary endpoints. Potential reasons for the lack of treatment difference are the circulating virus in the community during this trial and the single dose design. All seasonal influenza A H1N1 viruses tested had the H275Y mutation. Current data supports the conclusion that a single administration of Peramivir will not have adequate activity against viruses with H275Y substitution. No clinical data are available on the development of resistance to Peramivir.
http://www.cdc.gov/h1n1flu/eua/Final%20HCP%20Fa…
Joe,
Finally found the trial result data regarding peramivir.
It's tucked away in the Peramivir EUA, Fact Sheet for HCP published a few days ago. I'd like your opinion on the FDA playing hard ball with GSK and not persuading GSK to produce iv zanamivir (a special patent extension for iv zanamivir to make it worth GSK's while to fund trials- after all GSK got screwed over by the FDA and Tamiflu fast-tracking).
“At the onset of the current H1N1 outbreak, FDA contacted GSK about the appropriate regulatory mechanism to provide nebulized zanamivir for U.S. patients on a compassionate-use basis. GSK determined the most expedient approach is through an emergency IND, in which the individual-treating clinician acts as the principal investigator for a single patient. GSK has documentation based on limited data on how to treat. However, the supply of nebulized zanamivir is extremely limited. It was manufactured when GSK was actively developing the IV formulation. GSK feels it should be available worldwide for compassionate-use provided that appropriate regulatory mechanisms are in place. The remaining clinical trial material has been earmarked for compassionate-use. GSK has no intention to restart the manufacturing of zanamivir solution.”
“Following its meeting with FDA, and after reviewing the recent FDA draft guidance, specifically the guidance on clinical trial design in severe hospitalized patients, GSK sees no feasible path to registration; therefore IV-zanamivir remains on hold. GSK will consider a clinical development proposal from a third party for influenza antiviral drug development if it includes a clinical development plan that is reasonable, feasible, scientifically robust, and likely to lead to registration and approval. If the clinical development plan does not meet these criteria, GSK would not support a trial, execute the trial, or provide the drug. However, GSK would not prohibit a third party from sourcing the product from another licensed manufacturer.”
http://www.hhs.gov/aspr/conferences/nbsb/nbsb-h…
What a poor second choice iv peramivir is,
“”In another phase 2 trial (Trial 212) of intramuscular Peramivir 600 mg compared to placebo in patients with acute uncomplicated influenza, a statistically significant treatment effect was not observed for the primary endpoint (time to alleviation of symptoms) or secondary endpoints. Potential reasons for the lack of treatment difference are the circulating virus in the community during this trial and the single dose design. All seasonal influenza A H1N1 viruses tested had the H275Y mutation. Current data supports the conclusion that a single administration of Peramivir will not have adequate activity against viruses with H275Y substitution. No clinical data are available on the development of resistance to Peramivir.
http://www.cdc.gov/h1n1flu/eua/Final%20HCP%20Fa…
What about Peramivir developed by Biocryst?
Very encouraging PII results… with 100x drug concentration vs Tamiflu. It's in P III trials in Japan done by Shionogi.